Quorum's Dedication to Transforming Cancer Care
Here at Quorum Innovations, we’re committed to breaking new ground in the treatment of people dealing with glioblastoma multiforme (GBM), an extremely difficult condition. Our goal with IGV-001, our the latest immunotherapy candidate, is to completely transform the way this aggressive kind of brain cancer is treated.

“Our highest priority and motivation are the patients, families and caregivers.”
David Andrews, M.D.
Co-Founder and Chief Medical Officer
Harnessing Decades of Research for Personalized Therapies
Years of research and development have gone into creating our flagship product, IGV-001, which is intended to use a patient’s own immune system to its maximum capacity in the fight against GBM. Our goal is to provide patients a customized and efficient course of treatment by binding the whole antigen profile of the tumor and inducing both innate and adaptive immune responses.
Advancing Clinical Trials to Improve Patient Outcomes
We are pleased to report that individuals with newly diagnosed GBM will now be able to participate in a Phase 2b clinical study for IGV-001. Building on the encouraging outcomes of our Phase 1 trial, this trial will assess our novel therapy’s safety and effectiveness in a broader patient group. We seek to raise the bar for GBM patient care and increase survival rates via a dedication to thorough research and patient-centered treatment.
Phase 1 Studies in ndGBM
What Is The Purpose Of A Phase 2 Trial?
Phase 2 trials evaluate the safety and effectiveness of a drug, typically using the same dosage and schedule established in Phase 1 but in a larger group of patients. The goal is to further understand how well the drug works and its safety profile.
How Is The Phase 2 Study Designed?
Building on positive Phase 1 results, our Phase 2b study with IGV-001 involves 93 newly diagnosed GBM patients. Biodiffusion chambers are placed post-surgery, with one-third receiving a placebo and two-thirds receiving IGV-001. The study is blinded, ensuring unbiased evaluation.
What Are The Requirements For Enrolling In The Phase 2 Study?
Patients must have newly diagnosed GBM before starting chemotherapy or radiation to qualify for our Phase 2b study. Additional eligibility criteria, including general health and medication usage, will be discussed with your doctor and research team.
What Happens If I’m Interested In Participating?
After thorough discussion of study details, risks, and benefits, participants sign an informed consent form. Withdrawal is possible at any time. Tests, such as scans and blood tests, determine eligibility. Surgery for brain tumor removal follows upon satisfactory test results.
How Is Eligibility Determined?
Upon consent, the research team schedules and conducts tests like scans and blood tests to determine eligibility. Once tests confirm eligibility and the participant signs consent, brain tumor surgery is scheduled. If deemed ineligible, alternative treatment options are explored.
Phase 2b Clinical Study in ndGBM
What Is The Purpose Of A Phase 2 Clinical Trial?
Phase 2 trials evaluate the safety and effectiveness of a drug established as safe in Phase 1, but in a larger group of patients. The goal is to further assess the drug’s safety and efficacy in treating the targeted condition.
Can You Explain The Design Of The Phase 2 Study?
Building on Phase 1 success, our Phase 2b trial with IGV-001 involves 93 newly diagnosed GBM patients. Biodiffusion chambers are placed post-surgery, with a portion receiving a placebo. The study is blinded, ensuring unbiased evaluation of treatment outcomes.
What Are The Eligibility Criteria For Enrolling In The Phase 2 Study?
Patients must have newly diagnosed GBM and not have started chemotherapy or radiation to qualify for our Phase 2b trial. Additional eligibility criteria, such as overall health and medication use, will be discussed with your healthcare team.
How Do I Participate In The Phase 2 Trial?
After thorough discussion of study details, risks, and benefits, participants sign an informed consent form. Withdrawal is possible at any time. Eligibility is determined through various tests, including scans and blood tests, followed by brain tumor surgery scheduling.
How Is Eligibility Determined?
Upon consent, the research team conducts tests like scans and blood tests to determine eligibility. Once tests confirm eligibility and consent is given, brain tumor surgery is scheduled. If deemed ineligible, alternative treatment options are explored with the patient’s healthcare team.
Expanded Access Policy
For information about Quorum’s Expanded Access Policy, please click here.
“We are excited about the potential of IGV-001 for the treatment of ndGBM and look forward to fully enrolling this trial and seeing its results.”
Jill Krause
Vice President, Head of Clinical Operations

What does a Phase 2 trial investigate?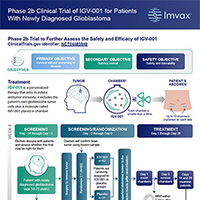
Phase 2 trials generally utilize the same dose and schedule of a drug determined to be safe and effective in Phase 1, but in a larger group of patients. The goals of a Phase 2 trial are to further determine the safety of a new drug and to evaluate its effectiveness. A summary of this study is available in infographic format by clicking on the image.
What is the design of the Phase 2 study?
Based on the encouraging results in the Phase 1 studies, Imvax has initiated a Phase 2b study with IGV-001 in 93 patients with ndGBM. The trial will be run at several cancer centers, generally in the Eastern half of the United States. All patients will have biodiffusion chambers placed in the abdomen after surgery for the brain tumor. One-third (1/3) of the patients will have a placebo (inactive drug) in the biodiffusion chambers, and two-thirds (2/3) will have active IGV-001 in the biodiffusion chambers. The study is blinded, meaning participants, doctors, and treatment teams will not know if they are receiving IGV-001 or the placebo. In rare cases, treatment can be revealed (unblinded) if doctors feel that information is important to know for additional medical care or an emergency. All patients will receive standard chemotherapy (temozolomide) with radiation followed by chemotherapy (temozolomide) alone after recovery from brain tumor surgery, just as was done in the Phase 1 study.
What are the requirements to enroll in the Phase 2 study?
The primary requirement for eligibility in our Phase 2b study is that patients must have newly diagnosed GBM prior to starting chemotherapy or radiation. Other important requirements for patient eligibility in the study, such as general health and other medications, will be reviewed and discussed with doctors and research teams. Most, but not all, patients with ndGBM will be eligible for this study.
What happens if I’m interested in enrolling?
Once the study details, risks, benefits and other treatment options have been discussed with participants, they will be asked to sign an informed consent form, acknowledging that they are willing to proceed with the study. Patients can withdraw their consent to participate in the study at any time and for any reason. Procedures that they may be financially responsible for will also be discussed, although in most cases these procedures can be billed to the patient’s insurance company.
How is my eligibility determined?
Once participants sign the consent form, their research team will begin scheduling and performing various tests required to determine their eligibility for the study. These will include items such as scans, blood tests and tests on their heart. Once all of these tests are performed and the results are satisfactory, patients will be scheduled for surgery on their brain tumor. Treatment teams will provide a schedule for all of these. If a patient is determined to not be eligible for the study, their treatment team will review other treatment options.
Where is the study taking place?
You can find the study listing (NCT04485949) in www.clinicaltrials.gov. The following sites are participating in the study:
- Dartmouth Hitchcock Medical Center, Lebanon, NH
- Tufts Medical Center, Boston, MA
- Rhode Island Hospital, Providence, RI
- Lenox Hill Hospital, New York, NY
- Montefiore Medical Center, New York, NY
- Icahn School of Medicine at Mount Sinai, New York, NY
- Columbia University, New York, NY
- Weill Cornell Medicine, New York, NY
- Westchester Medical Center, Valhalla, NY
- Memorial Sloan Kettering Cancer Center, New York, NY
- Northwell Health at North Shore University Hospital, Manhasset, NY
- Jersey Shore University Medical Center, Neptune, NJ
- Thomas Jefferson University, Philadelphia, PA
- University of Pennsylvania, Philadelphia, PA
- The Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA
- University of North Carolina, Chapel Hill, NC
- Henry Ford Health System, Detroit, MI
- The Ohio State University, Columbus, OH
- University of Wisconsin, Madison, WI
- Mayo Clinic, Jacksonville, FL
What does a Phase 2 trial investigate?
Phase 2 trials generally utilize the same dose and schedule of a drug determined to be safe and effective in Phase 1, but in a larger group of patients. The goals of a Phase 2 trial are to further determine the safety of a new drug and to evaluate its effectiveness. A summary of this study is available in infographic format by clicking on the image.
What is the design of the Phase 2 study?
Based on the encouraging results in the Phase 1 studies, Imvax has initiated a Phase 2b study with IGV-001 in 93 patients with ndGBM. The trial will be run at several cancer centers, generally in the Eastern half of the United States. All patients will have biodiffusion chambers placed in the abdomen after surgery for the brain tumor. One-third (1/3) of the patients will have a placebo (inactive drug) in the biodiffusion chambers, and two-thirds (2/3) will have active IGV-001 in the biodiffusion chambers. The study is blinded, meaning participants, doctors, and treatment teams will not know if they are receiving IGV-001 or the placebo. In rare cases, treatment can be revealed (unblinded) if doctors feel that information is important to know for additional medical care or an emergency. All patients will receive standard chemotherapy (temozolomide) with radiation followed by chemotherapy (temozolomide) alone after recovery from brain tumor surgery, just as was done in the Phase 1 study.
What are the requirements to enroll in the Phase 2 study?
The primary requirement for eligibility in our Phase 2b study is that patients must have newly diagnosed GBM prior to starting chemotherapy or radiation. Other important requirements for patient eligibility in the study, such as general health and other medications, will be reviewed and discussed with doctors and research teams. Most, but not all, patients with ndGBM will be eligible for this study.
What happens if I’m interested in enrolling?
Once the study details, risks, benefits and other treatment options have been discussed with participants, they will be asked to sign an informed consent form, acknowledging that they are willing to proceed with the study. Patients can withdraw their consent to participate in the study at any time and for any reason. Procedures that they may be financially responsible for will also be discussed, although in most cases these procedures can be billed to the patient’s insurance company.
How is my eligibility determined?
Once participants sign the consent form, their research team will begin scheduling and performing various tests required to determine their eligibility for the study. These will include items such as scans, blood tests and tests on their heart. Once all of these tests are performed and the results are satisfactory, patients will be scheduled for surgery on their brain tumor. Treatment teams will provide a schedule for all of these. If a patient is determined to not be eligible for the study, their treatment team will review other treatment options.
Where is the study taking place?
You can find the study listing (NCT04485949) in www.clinicaltrials.gov. The following sites are participating in the study:
- Dartmouth Hitchcock Medical Center, Lebanon, NH
- Tufts Medical Center, Boston, MA
- Rhode Island Hospital, Providence, RI
- Lenox Hill Hospital, New York, NY
- Montefiore Medical Center, New York, NY
- Icahn School of Medicine at Mount Sinai, New York, NY
- Columbia University, New York, NY
- Weill Cornell Medicine, New York, NY
- Westchester Medical Center, Valhalla, NY
- Memorial Sloan Kettering Cancer Center, New York, NY
- Northwell Health at North Shore University Hospital, Manhasset, NY
- Jersey Shore University Medical Center, Neptune, NJ
- Thomas Jefferson University, Philadelphia, PA
- University of Pennsylvania, Philadelphia, PA
- The Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA
- University of North Carolina, Chapel Hill, NC
- Henry Ford Health System, Detroit, MI
- The Ohio State University, Columbus, OH
- University of Wisconsin, Madison, WI
- Mayo Clinic, Jacksonville, FL
Patients and Community Resources
Clinical trials are highly regulated by federal regulatory agencies. You can find additional information at the website of The U.S. Food and Drug Administration.
Additional information can be found in the following websites:
- American Cancer Society (ACS)
- National Cancer Institute (NCI)
- National Comprehensive Cancer Network (NCCN)
- ClinicalTrials.gov
Quorum is proud to be a sponsor of the following patient advocacy organizations that are providing critical funding and advocacy for brain cancer research and much-needed support for patients and their families. Click on the links below to learn more.
Contact us
Eva Berkes, MD, Co-Founder
Nicholas Monsul, MD, Co-Founder
941-951-0126
evaberkesmd@quoruminnovations.com
nickmonsulmd@quoruminnovations.com
